- Product Details
Keywords
- 9 Mix and Fluconazole in acetonitrile
- 9 Mix and Fluconazole
- 9 Mix
Quick Details
- ProName: 9 Mix and Fluconazole in acetonitrile
- Appearance: 1.2mL
- Application: Laboratory analysisInstrument calibrat...
- DeliveryTime: 5-10 Days
- PackAge: Ampoule bottle
- Port: China
- ProductionCapacity: /
- Purity: 100μgmL
- Transportation: By Air
- LimitNum: 0
Superiority
This product can be used as a working standard for daily analysis and testing; It can also be used for laboratory quality control such as test method evaluation and instrument calibration.
一、Traceability and value determination method
The standard value is verified by . The traceability of the product quantity value is ensured through the use of production, measurement methods and measuring instruments that meet the requirements of metrological characteristics.
二、Characteristic value and expanded uncertainty
| No | Analyte | CAS | Standard value | Uncertainty(k=2) | Matrix |
|---|---|---|---|---|---|
| 1 | (+)-Griseofulvin | 126-07-8 | 100μg/mL | 4% | Acetonitrile |
| Ketoconazole | 65277-42-1 | 100μg/mL | 4% | ||
| Clotrimazole | 23593-75-1 | 100μg/mL | 4% | ||
| Econazole | 27220-47-9 | 100μg/mL | 4% | ||
| Miconazole | 22916-47-8 | 100μg/mL | 4% | ||
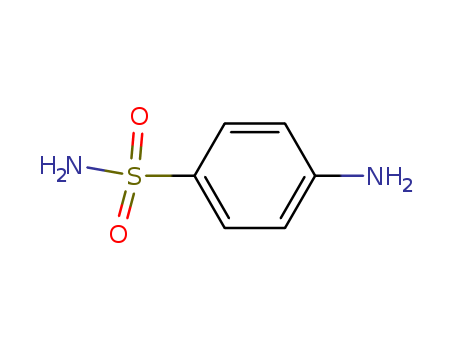
| Fluconazole | 86386-73-4 | 100μg/mL | 4% | ||
| Bifonazole | 60628-96-8 | 100μg/mL | 4% | ||
| Ciclopirox ethanolamine | 41621-49-2 | 100μg/mL | 4% | ||
| Naftifine | 65472-88-0 | 100μg/mL | 4% |
三、Uniformity test and stability investigation
According to ISO 33405 metrology technical specification, the samples are randomly sampled after packaging, and the uniformity test and long-term stability tracking of the product are carried out by . The results show that the uniformity meets the F test rules and has good stability.
The quantitative value of this product is valid for 6 months from the date of value determination. The research and development unit will continue to track and monitor the stability of the product. If the value changes during the validity period, the user will be notified in time.
四、Packaging storage and use
Packaging: This product is packaged in Ampoule bottle, with the specification of 1.2mL. It should be protected from breaking when carried or transported.
Storage: and in the sealed and light-proof conditions. Before use, it should be fully shaken to ensure homogeneity. This product should be used as soon as possible after being opened. During the use process, strict precautions should be taken to prevent contamination.
Details
This product can be used as a working standard for daily analysis and testing; It can also be used for laboratory quality control such as test method evaluation and instrument calibration.
一、Traceability and value determination method
The standard value is verified by . The traceability of the product quantity value is ensured through the use of production, measurement methods and measuring instruments that meet the requirements of metrological characteristics.
二、Characteristic value and expanded uncertainty
| No | Analyte | CAS | Standard value | Uncertainty(k=2) | Matrix |
|---|---|---|---|---|---|
| 1 | (+)-Griseofulvin | 126-07-8 | 100μg/mL | 4% | Acetonitrile |
| Ketoconazole | 65277-42-1 | 100μg/mL | 4% | ||
| Clotrimazole | 23593-75-1 | 100μg/mL | 4% | ||
| Econazole | 27220-47-9 | 100μg/mL | 4% | ||
| Miconazole | 22916-47-8 | 100μg/mL | 4% | ||
| Fluconazole | 86386-73-4 | 100μg/mL | 4% | ||
| Bifonazole | 60628-96-8 | 100μg/mL | 4% | ||
| Ciclopirox ethanolamine | 41621-49-2 | 100μg/mL | 4% | ||
| Naftifine | 65472-88-0 | 100μg/mL | 4% |
三、Uniformity test and stability investigation
According to ISO 33405 metrology technical specification, the samples are randomly sampled after packaging, and the uniformity test and long-term stability tracking of the product are carried out by . The results show that the uniformity meets the F test rules and has good stability.
The quantitative value of this product is valid for 6 months from the date of value determination. The research and development unit will continue to track and monitor the stability of the product. If the value changes during the validity period, the user will be notified in time.
四、Packaging storage and use
Packaging: This product is packaged in Ampoule bottle, with the specification of 1.2mL. It should be protected from breaking when carried or transported.
Storage: and in the sealed and light-proof conditions. Before use, it should be fully shaken to ensure homogeneity. This product should be used as soon as possible after being opened. During the use process, strict precautions should be taken to prevent contamination.


 Premiumsupplier
Premiumsupplier